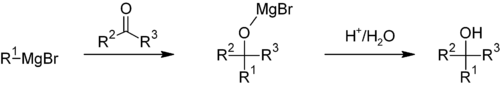Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides (Grignard reagents) act as nucleophiles and attack electrophilic carbon atoms that are present within polar bonds (e.g. in a carbonyl group as in the example shown below) to yield a carbon-carbon bond, thus altering hybridization about the reaction center.[1] The Grignard reaction is an important tool in the formation of carbon-carbon bonds[2][3] and for the formation of carbon-phosphorus, carbon-tin, carbon-silicon, carbon-boron and other carbon-heteroatom bonds.
The nucleophilic organometallic addition reaction is irreversible due to the high pKa value of the alkyl component (pKa = ~45). Such reactions are not ionic; the Grignard reagent exists as an organometallic cluster (in ether).
The disadvantage of Grignard reagents is that they readily react with protic solvents (such as water), or with functional groups with acidic protons, such as alcohols and amines. In fact, atmospheric humidity in the lab can dictate one's success when trying to synthesize a Grignard reagent from magnesium turnings and an alkyl halide. One of many methods used to exclude water from the reaction atmosphere is to flame-dry the reaction vessel to evaporate all moisture, which is then sealed to prevent moisture from returning. However, though the reagents still need to be dry, ultrasound can allow Grignard reagents to form with less stringent regard to the amount of water in the reaction mix by activating the surface of the magnesium such that it consumes any water present.[4]
Another disadvantage of Grignard reagents is that they do not readily form carbon-carbon bonds by reacting with alkyl halides via an SN2 mechanism.
Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France) who was awarded the 1912 Nobel Prize in Chemistry for this work.
Contents |
Reaction mechanism
The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state.[5]
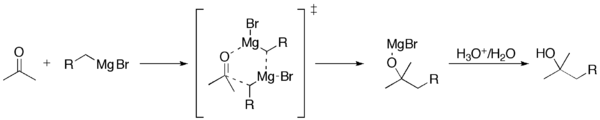
However, with hindered Grignard reagents, the reaction may proceed by single-electron transfer.
In a reaction involving Grignard reagents, it is important to ensure that no water is present, which would otherwise cause the reagent to rapidly decompose. Thus, most Grignard reactions occur in solvents such as anhydrous diethyl ether or tetrahydrofuran, because the oxygen of these solvents stabilizes the magnesium reagent. The reagent may also react with oxygen present in the atmosphere, inserting an oxygen atom between the carbon base and the magnesium halide group. Usually, this side-reaction may be limited by the volatile solvent vapors displacing air above the reaction mixture. However, it may be preferable for such reactions to be carried out in nitrogen or argon atmospheres, especially for smaller scales.
Synthesis of Grignard reagents
Grignard reagents are formed via the action of an alkyl or aryl halide on magnesium metal.[6] The reaction is conducted by adding the organic halide to a suspension of magnesium in an ether, which provides ligands required to stabilize the organomagnesium compound. Typical solvents are diethyl ether and tetrahydrofuran. Oxygen and protic solvents such as water or alcohols are not compatible with Grignard reagents. The reaction proceeds through single electron transfer. [7] [8] [9]
- R−X + Mg → R−X•− + Mg•+
- R−X•− → R• + X−
- X− + Mg•+ → XMg•
- R• + XMg• → RMgX
Grignard reactions often start slowly. As is common for reactions involving solids and solution, initiation follows an induction period during which reactive magnesium becomes exposed to the organic reagents. After this induction period, the reactions can be highly exothermic. Alkyl and aryl bromides and iodides are common substrates. Chlorides are also used, but fluorides are generally unreactive, except with specially activated magnesium, such as Rieke magnesium.
Many Grignard reagents, e.g. methylmagnesium chloride, phenylmagnesium bromide, and allylmagnesium bromide are available commercially in tetrahydrofuran or diethyl ether solutions.
Via the Schlenk equilibrium, Grignard reagents form varying amounts of diorganomagnesium compounds (R = organic group, X = halide):
- 2 RMgX
 R2Mg + MgX2
R2Mg + MgX2
Initiation
Many methods have been developed to initiate sluggish Grignard reactions. Mechanical methods include crushing of the Mg pieces in situ, rapid stirring, and sonication of the suspension. Iodine, methyl iodide, and 1,2-dibromoethane are commonly employed activating agents. The use of 1,2-dibromoethane is particularly advantageous as its action can be monitored by the observation of bubbles of ethylene. Furthermore, the side-products are innocuous:
- Mg + BrC2H4Br → C2H4 + MgBr2
The amount of Mg consumed by these activating agents is usually insignificant.
The addition of a small amount of mercuric chloride will amalgamate the surface of the metal, allowing it to react.
These methods weaken the passivating layer of MgO, thereby exposing highly reactive magnesium to the organic halide.
Reactions of Grignard reagents
Reactions with carbonyl compounds
Grignard reagents will react with a variety of carbonyl derivatives.[10]
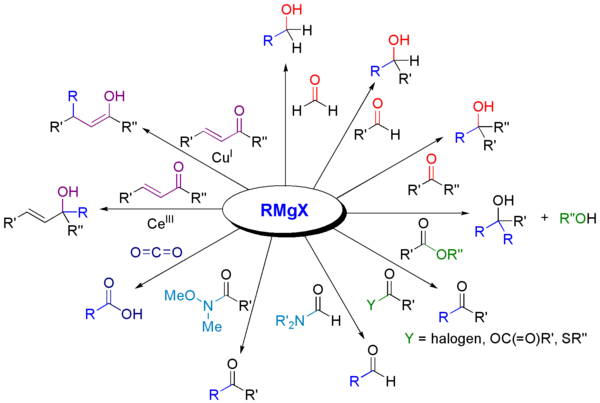
The most common application is for alkylation of aldehydes and ketones, as in this example:[11]
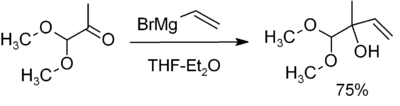
.
Note that the acetal function (a masked carbonyl) does not react.
Such reactions usually involve an aqueous acidic workup, though this is rarely shown in reaction schemes. In cases where the Grignard reagent is adding to a prochiral aldehyde or ketone, the Felkin-Anh model or Cram's Rule can usually predict which stereoisomer will be formed.
Reactions with other electrophiles
In addition, Grignard reagents will react with other various electrophiles.
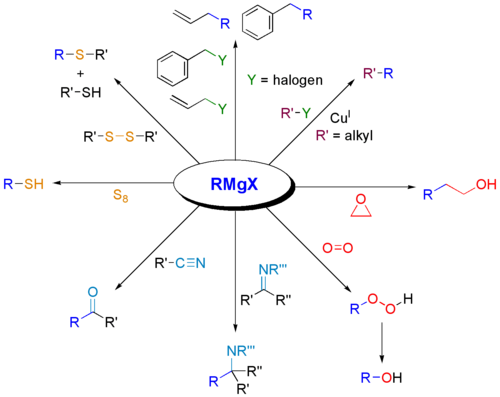
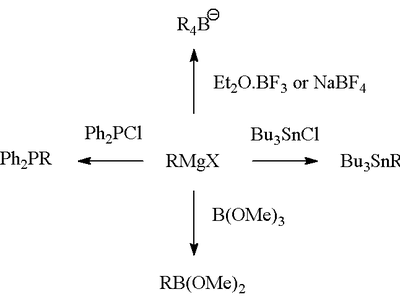
Formation of bonds to B, Si, P, Sn
Also the Grignard reagent is very useful for forming carbon-heteroatom bonds.
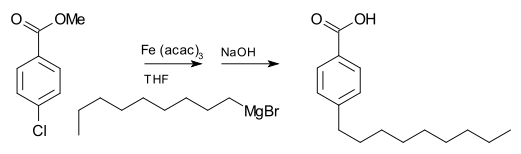
Carbon-carbon coupling reactions
A Grignard reagent can also be involved in coupling reactions. For example, nonylmagnesium bromide reacts with an aryl chloride to a nonyl benzoic acid, in the presence of iron(III) acetylacetonate. Ordinarily, the Grignard reagent will attack the ester over the aryl halide.[12]
For the coupling of aryl halides with aryl Grignards, nickel chloride in THF is also a good catalyst. Additionally, an effective catalyst for the couplings of alkyl halides is dilithium tetrachlorocuprate (Li2CuCl4), prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl2) in THF. The Kumada-Corriu coupling gives access to styrenes.
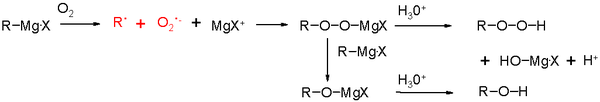
Oxidation
The oxidation of a Grignard reagent with oxygen takes place through a radical intermediate to a magnesium hydroperoxide. Hydrolysis of this complex yields hydroperoxides and reduction with an additional equivalent of Grignard reagent gives an alcohol.
The synthetic utility of Grignard oxidations can be increased by a reaction of Grignards with oxygen in presence of an alkene to an ethylene extended alcohol.[13] This modification requires aryl or vinyl Grignards. Adding just the Grignard and the alkene does not result in a reaction demonstrating that the presence of oxygen is essential. Only drawback is the requirement of at least two equivalents of Grignard although this can partly be circumvented by the use of a dual Grignard system with a cheap reducing Grignard such as n-butylmagnesium bromide.
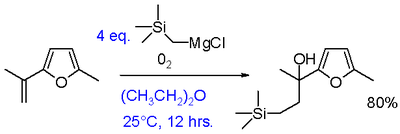
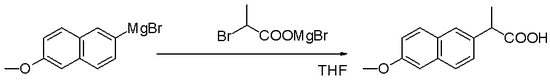
Nucleophilic aliphatic substitution
Grignard reagents are nucleophiles in nucleophilic aliphatic substitutions for instance with alkyl halides in a key step in industrial Naproxen production:
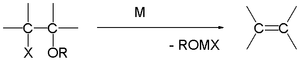
Elimination
In the Boord olefin synthesis, the addition of magnesium to certain β-haloethers results in an elimination reaction to the alkene. This reaction can limit the utility of Grignard reactions.
Grignard degradation
Grignard degradation [14][15] at one time was a tool in structure elucidation in which a Grignard RMgBr formed from a heteroaryl bromide HetBr reacts with water to Het-H (bromine replaced by a hydrogen atom) and MgBrOH. This hydrolysis method allows the determination of the number of halogen atoms in an organic compound. In modern usage Grignard degradation is used in the chemical analysis of certain triacylglycerols.[16]
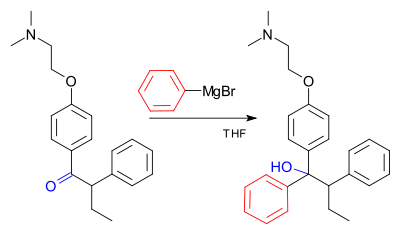
Industrial use
An example of the Grignard reaction is a key step in the industrial production of Tamoxifen[17] (currently used for the treatment of estrogen receptor positive breast cancer in women)[18] :
See also
- Wittig reaction
- Barbier reaction
- Bodroux-Chichibabin aldehyde synthesis
- Fujimoto-Belleau reaction
- Organolithium reagents
- Sakurai reaction
References
- ↑ Grignard, V. (1900), "Sur quelques nouvelles combinaisons organométaliques du magnésium et leur application à des synthèses d'alcools et d'hydrocabures", Compt. Rend. 130: 1322–1325, http://gallica.bnf.fr/ark:/12148/bpt6k3086n/f1322.table
- ↑ Shirley, D. A. (1954), Org. React 8: 28–58
- ↑ Huryn, D. M. (1991), Comp. Org. Syn 1: 49–75
- ↑ Smith, David H. (1999), "Grignard Reactions in "Wet" Ether", Journal of Chemical Education 76: 1427, doi:10.1021/ed076p1427
- ↑ Maruyama, K.; Katagiri, T. (1989), "Mechanism of the Grignard reaction", J. Phys. Org. Chem 2: 205, doi:10.1002/poc.610020303
- ↑ Lai Yee Hing (1981), "Grignard Reagents from Chemically Activated Magnesium", Synthesis 1981: 585–604, doi:10.1055/s-1981-29537
- ↑ Garst, J. F.; Ungvary, F. "Mechanism of Grignard reagent formation". In Grignard Reagents; Richey, R. S., Ed.; John Wiley & Sons: New York, 2000; pp 185–275. ISBN 0-471-99908-3.
- ↑ Advanced Organic chemistry Part B: Reactions and Synthesis F.A. Carey, R.J. Sundberg 2nd Ed. 1983
- ↑ doi:10.1021/ja00521a034
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Henry Gilman and R. H. Kirby (1941), "Butyric acid, α-methyl-", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0361; Coll. Vol. 1: 361
- ↑ Haugan, Jarle André; Songe, Pål; Rømming, Christian; Rise, Frode; Hartshorn, Michael P.; Merchán, Manuela; Robinson, Ward T.; Roos, Björn O. et al. (1997), "Total Synthesis of C31-Methyl Ketone Apocarotenoids 2: The First Total Synthesis of (3R)-Triophaxanthin.", Acta Chimica Scandinavica 51: 1096–1103, doi:10.3891/acta.chem.scand.51-1096, http://actachemscand.dk/pdf/acta_vol_51_p1096-1103.pdf, retrieved 2009-11-26
- ↑ A. Fürstner, A. Leitner, G. Seidel (2004), "4-Nonylbenzoic Acid", Org. Synth. 81: 33–42, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v81p0033
- ↑ Youhei Nobe, Kyohei Arayama, and Hirokazu Urabe (2005), "Air-Assisted Addition of Grignard Reagents to Olefins. A Simple Protocol for a Three-Component Coupling Process Yielding Alcohols", J. Am. Chem. Soc. 127 (51): 18006–18007, doi:10.1021/ja055732b
- ↑ Steinkopf, Wilhelm; Jacob, Hans; Penz, Herbert (1934), "Studien in der Thiophenreihe. XXVI. Isomere Bromthiophene und die Konstitution der Thiophendisulfonsäuren", Justus Liebig s Annalen der Chemie 512: 136, doi:10.1002/jlac.19345120113
- ↑ Steinkopf, Wilhelm; V. Petersdorff, Hans-JüRgen (1940), "Studien in der Thiophenreihe. LI. Atophanartige Derivate des Dithienyls und Diphenyls", Justus Liebig s Annalen der Chemie 543: 119, doi:10.1002/jlac.19405430110
- ↑ Myher JJ, Kuksis A (February 1979), "Stereospecific analysis of triacylglycerols via racemic phosphatidylcholines and phospholipase C", Can. J. Biochem. 57 (2): 117–24, doi:10.1139/o79-015, PMID 455112
- ↑ "Grignard Reagents: New Developments", ISBN: 0–471
- ↑ Jordan VC (1993), "Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer", Br J Pharmacol 110 (2): 507–17, PMID 8242225
Further reading
- ed. by Gary S. Silverman .... (1996), Rakita, Philip E.; Silverman, Gary, ed., Handbook of Grignard reagents, New York, N.Y: Marcel Dekker, ISBN 0-8247-9545-8
Gallery
 Magnesium turnings placed on a flask. |
 Covered with THF and a small piece of iodine added. |
 A solution of alkyl bromide was added while heating. |
 After completion of the addition, the mixture was heated for a while. |
 Formation of the Grignard reagent had completed. A small amount of magnesium still remained in the flask. |
 The Grignard reagent thus prepared was cooled to 0°C before the addition of carbonyl compound. The solution became cloudy since the Grignard reagent precipitated out. |
 A solution of carbonyl compound was added to the Grignard reagent. |
 The solution was warmed to room temperature. The reaction was complete. |
|
|||||||||||||||||